Are you searching for 'kinetics of the reactions between magnesium and acids biology essay'? Here you can find the questions and answers on the subject.
Table of contents
- Kinetics of the reactions between magnesium and acids biology essay in 2021
- Hydrochloric acid experiment
- How does concentration affect the rate of reaction between magnesium and hydrochloric acid
- Magnesium reacts with hydrochloric acid to produce hydrogen gas an experiment was set up
- Reaction of magnesium with hydrochloric acid lab answers
- Reaction of magnesium with dilute hydrochloric acid
- How to measure rate of reaction between magnesium and hydrochloric acid
- Hcl and magnesium ribbon reaction
Kinetics of the reactions between magnesium and acids biology essay in 2021
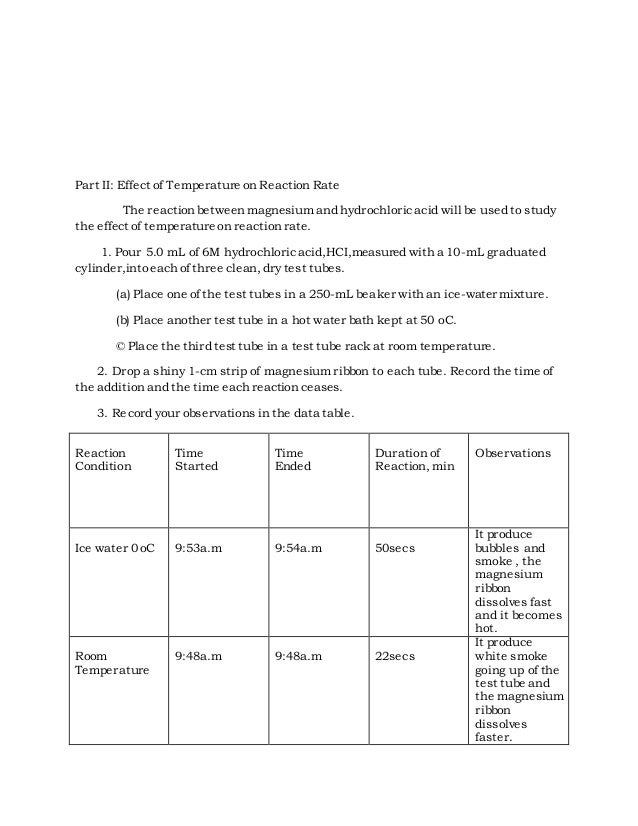 This image illustrates kinetics of the reactions between magnesium and acids biology essay.
This image illustrates kinetics of the reactions between magnesium and acids biology essay.
Hydrochloric acid experiment
 This image representes Hydrochloric acid experiment.
This image representes Hydrochloric acid experiment.
How does concentration affect the rate of reaction between magnesium and hydrochloric acid
 This picture representes How does concentration affect the rate of reaction between magnesium and hydrochloric acid.
This picture representes How does concentration affect the rate of reaction between magnesium and hydrochloric acid.
Magnesium reacts with hydrochloric acid to produce hydrogen gas an experiment was set up
 This picture demonstrates Magnesium reacts with hydrochloric acid to produce hydrogen gas an experiment was set up.
This picture demonstrates Magnesium reacts with hydrochloric acid to produce hydrogen gas an experiment was set up.
Reaction of magnesium with hydrochloric acid lab answers
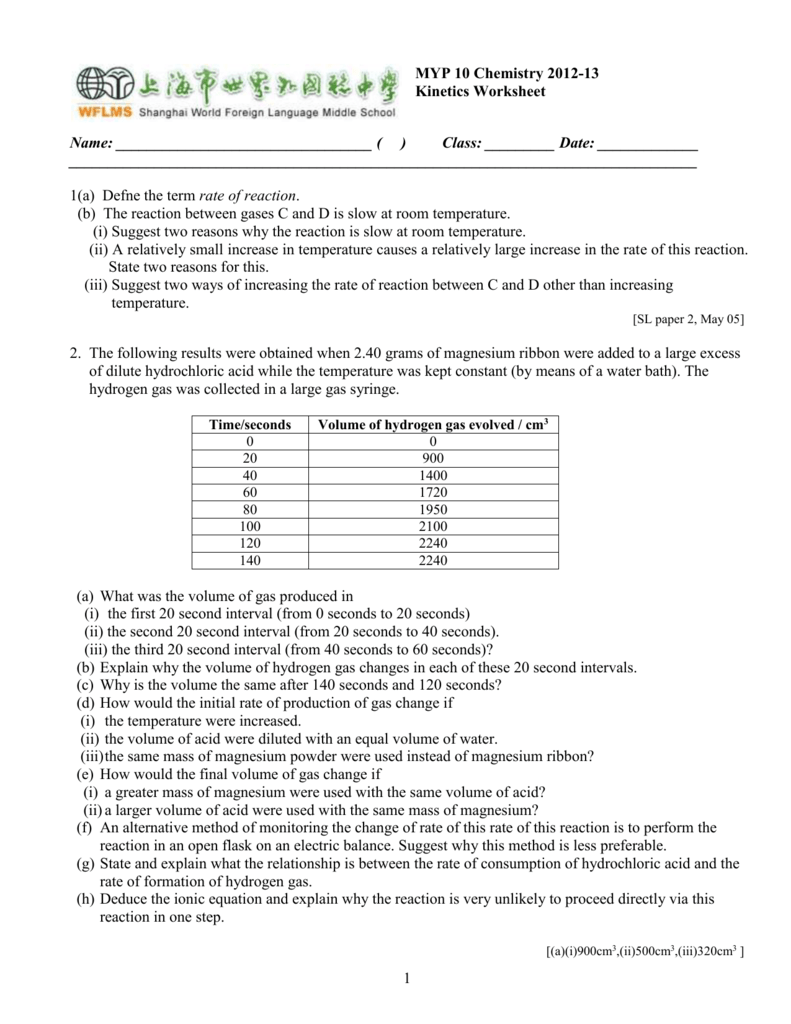 This picture demonstrates Reaction of magnesium with hydrochloric acid lab answers.
This picture demonstrates Reaction of magnesium with hydrochloric acid lab answers.
Reaction of magnesium with dilute hydrochloric acid
 This image demonstrates Reaction of magnesium with dilute hydrochloric acid.
This image demonstrates Reaction of magnesium with dilute hydrochloric acid.
How to measure rate of reaction between magnesium and hydrochloric acid
 This image demonstrates How to measure rate of reaction between magnesium and hydrochloric acid.
This image demonstrates How to measure rate of reaction between magnesium and hydrochloric acid.
Hcl and magnesium ribbon reaction
 This image demonstrates Hcl and magnesium ribbon reaction.
This image demonstrates Hcl and magnesium ribbon reaction.
What happens when molecules move with too little kinetic energy?
(3) Molecules moving too slowly, with too little kinetic energy, don’t react when they collide. The Activation energy, Ea, is the minimum energy required to initiate a chemical reaction. Ea is specific to a particular reaction. Arrhenius: Molecules must posses a minimum amount of energy to react.
How are the reactions between magnesium and acids?
Kinetics Of The Reactions Between Magnesium And Acids. HCl is a strong acid due to its full dissociation in water. This is very unstable (very reactive) as the negative change is only present on the chloride ion. Moving on to sulphuric acid, it consists of 2 hydrogen atoms resulting in two H+ ions (protons) forming,...
Which is faster 1.0 molar acid or magnesium acid?
The line showing the results of 1.0 molar acid is the steepest. The reaction is faster with stronger acid because it contains more acid particles. The greater number of acid particles the more chances of a collision between acid and magnesium particles therefore the faster the reaction.
How is the rate of reaction of magnesium with hydrochloric acid measured?
Magnesium reacts with dilute hydrochloric acid in a conical flask which is connected to an inverted measuring cylinder in a trough of water. The volume of hydrogen gas produced is measured over a few minutes, and the results are used to plot a graph
Last Update: Oct 2021